Oxygen compound top
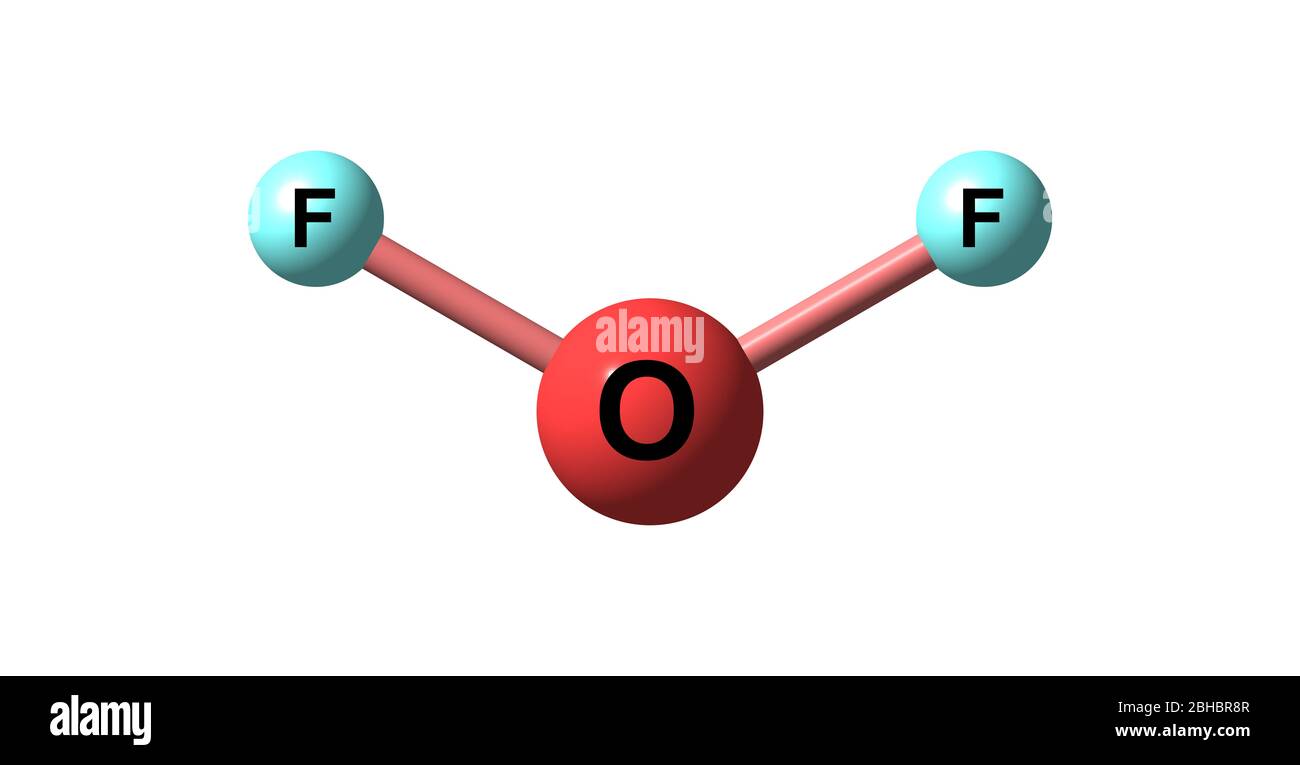
Oxygen compound top, Oxygen difluoride is the chemical compound with the formula OF2 top
$96.00
SAVE 50% OFF
$48.00
$0 today, followed by 3 monthly payments of $16.00, interest free. Read More
Oxygen compound top
Oxygen difluoride is the chemical compound with the formula OF2
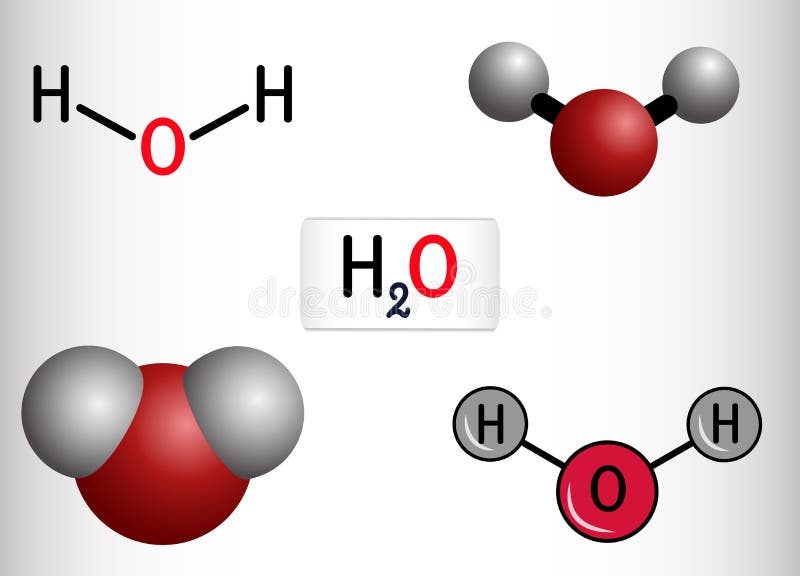
Water H2O HOH Molecule. it is Inorganic Hydroxy Compound
Oxygen Formula O2 Over 100 million chemical compounds CCDDS
When oxygen combines with any alkali metal M what is the formula
Chemical structures of compounds used in this study. Oxygen atoms
Naming compounds with Oxygen
Description
Product code: Oxygen compound top
18.9 Occurrence Preparation and Compounds of Oxygen Chemistry top, 18.9 Occurrence Preparation and Compounds of Oxygen Chemistry top, Compounds of Oxygen Encyclopedia MDPI top, Occurrence Preparation and Compounds of Oxygen Chemistry top, Oxygen compounds Wikipedia top, What is the name of the compound that has oxygen and hydrogen as top, Oxygen Discovery Symbol Properties Uses Facts Britannica top, Occurrence Preparation and Compounds of Oxygen Chemistry top, CH105 Chapter 9 Organic Compounds of Oxygen Chemistry top, Oxygen Properties Formulas Uses Lesson Study top, organic chemistry Addition of oxygen molecule to given aromatic top, Elements and Compounds are compared in the molecular structure top, 18.9 Occurrence Preparation and Compounds of Oxygen Chemistry top, What are common compounds of oxygen Quora top, Chemical structures of compounds used in this study. Oxygen atoms top, CH105 Chapter 9 Organic Compounds of Oxygen Chemistry top, Oxygen difluoride is the chemical compound with the formula OF2 top, Water H2O HOH Molecule. it is Inorganic Hydroxy Compound top, Oxygen Formula O2 Over 100 million chemical compounds CCDDS top, When oxygen combines with any alkali metal M what is the formula top, Chemical structures of compounds used in this study. Oxygen atoms top, Naming compounds with Oxygen top, What are common compounds of oxygen Quora top, WebElements Periodic Table Oxygen oxygen difluoride top, Oxygen O2 CID 977 PubChem top, Common Molecule Examples YourDictionary top, Molecule vs. Compound Definition Comparison Expii top, Water H2O HOH molecule. It is inorganic hydroxy compound top, Chemistry model of molecule water H2O scientific elements top, Water H2O HOH Molecule. it is Inorganic Hydroxy Compound top, Carbon dioxide CO2 is a naturally occurring chemical compound top, Oxygen splitting in compound 1. Color coding for atoms red top, About compounds of sulfur and oxygen MEL Chemistry top, Oxygen Difluoride Chemical Compound Formula Of2 Stock Illustration top, Carbon reacts with Oxygen to make two compounds. Compound A has 2.41 gramss of Carbon for each 3.22 top.
18.9 Occurrence Preparation and Compounds of Oxygen Chemistry top, 18.9 Occurrence Preparation and Compounds of Oxygen Chemistry top, Compounds of Oxygen Encyclopedia MDPI top, Occurrence Preparation and Compounds of Oxygen Chemistry top, Oxygen compounds Wikipedia top, What is the name of the compound that has oxygen and hydrogen as top, Oxygen Discovery Symbol Properties Uses Facts Britannica top, Occurrence Preparation and Compounds of Oxygen Chemistry top, CH105 Chapter 9 Organic Compounds of Oxygen Chemistry top, Oxygen Properties Formulas Uses Lesson Study top, organic chemistry Addition of oxygen molecule to given aromatic top, Elements and Compounds are compared in the molecular structure top, 18.9 Occurrence Preparation and Compounds of Oxygen Chemistry top, What are common compounds of oxygen Quora top, Chemical structures of compounds used in this study. Oxygen atoms top, CH105 Chapter 9 Organic Compounds of Oxygen Chemistry top, Oxygen difluoride is the chemical compound with the formula OF2 top, Water H2O HOH Molecule. it is Inorganic Hydroxy Compound top, Oxygen Formula O2 Over 100 million chemical compounds CCDDS top, When oxygen combines with any alkali metal M what is the formula top, Chemical structures of compounds used in this study. Oxygen atoms top, Naming compounds with Oxygen top, What are common compounds of oxygen Quora top, WebElements Periodic Table Oxygen oxygen difluoride top, Oxygen O2 CID 977 PubChem top, Common Molecule Examples YourDictionary top, Molecule vs. Compound Definition Comparison Expii top, Water H2O HOH molecule. It is inorganic hydroxy compound top, Chemistry model of molecule water H2O scientific elements top, Water H2O HOH Molecule. it is Inorganic Hydroxy Compound top, Carbon dioxide CO2 is a naturally occurring chemical compound top, Oxygen splitting in compound 1. Color coding for atoms red top, About compounds of sulfur and oxygen MEL Chemistry top, Oxygen Difluoride Chemical Compound Formula Of2 Stock Illustration top, Carbon reacts with Oxygen to make two compounds. Compound A has 2.41 gramss of Carbon for each 3.22 top.